Polymorph screening in pharmaceutical development - European Pharmaceutical Review
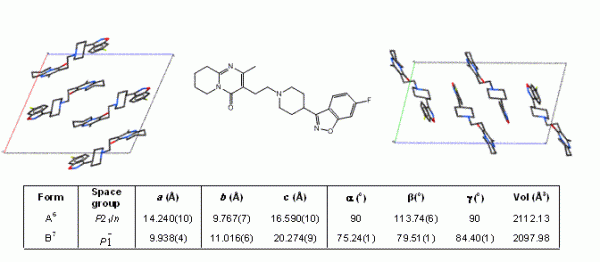
The majority of active pharmaceutical ingredients (APIs) are produced by crystallisation and so the phenomenon of polymorphism, whereby an organic molecule can adopt more than one crystalline form (Figure 1), is of considerable importance when trying to achieve consistent product quality during the manufacture of pharmaceutical solids and solid dosage forms. Although morphology and particle size-distribution are important solid-state characteristics, the uncontrolled occurrence of multiple physical forms (polymorphs, solvates, salts, co-crystals or amorphous) of an API can have significant effects on the performance of the material during processing, manufacture, storage and administration. For example, the solubility difference between some polymorphs has been shown to be over four times that of the least soluble form1 and can vary by significantly more for amorphous forms2.
Medicines, Free Full-Text
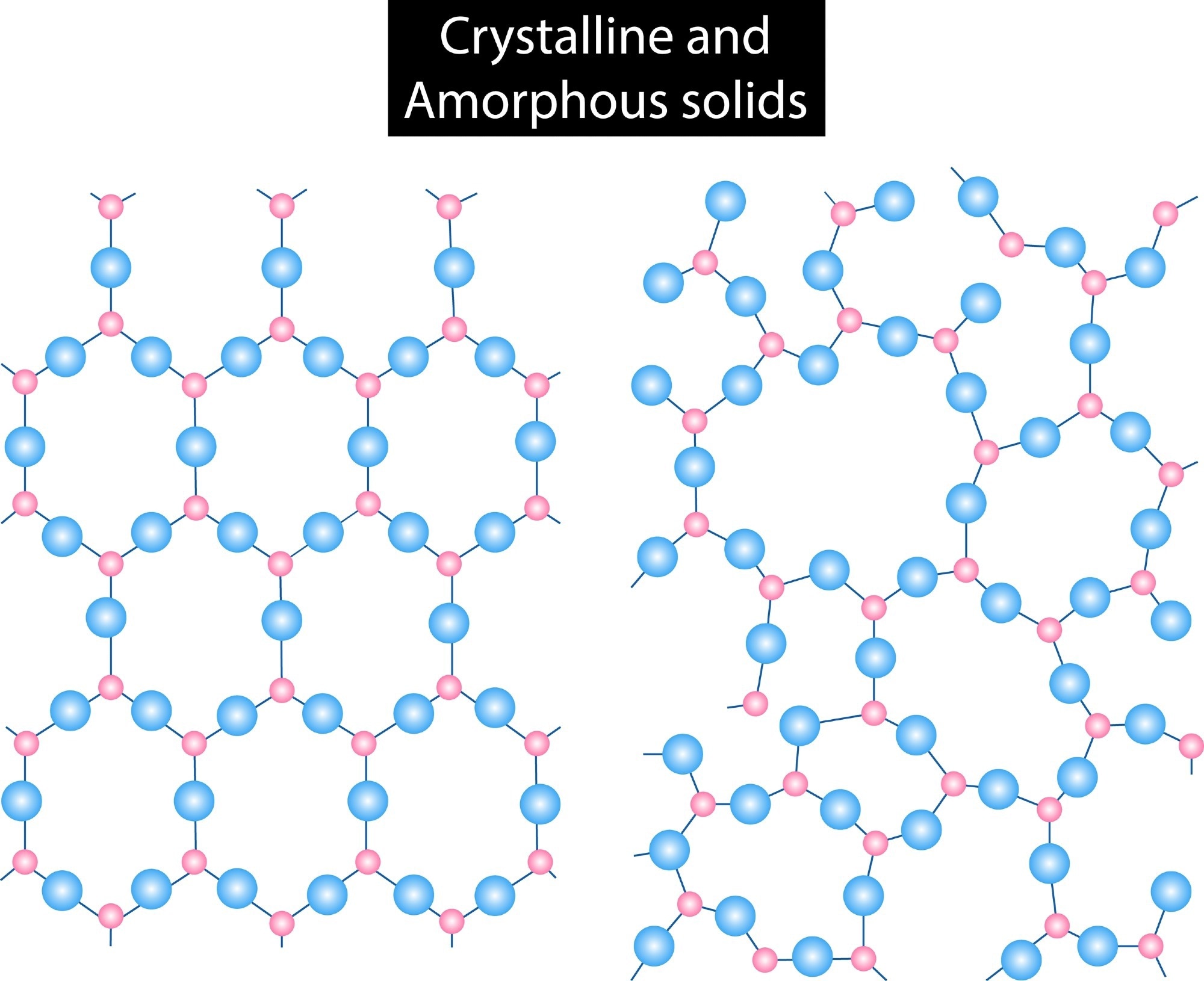
What are Polymorphs?

Control of Pharmaceutical Cocrystal Polymorphism on Various Scales

Trends in oral small-molecule drug discovery and product

Pharmaceutical cocrystals: A review of preparations

Opportunities and Challenges in Mechanochemical Cocrystallization
Frontiers Recent Advances in Pharmaceutical Cocrystals: From

What are Polymorphs?
A logical network-based drug-screening platform for Alzheimer's

Estimation of the Solubility of Metastable Polymorphs: A Critical

Efficient Polymorph Screening through Crystallization from Bulk

Transmission Low-Frequency Raman Spectroscopy for Quantification

Polymorph screening in pharmaceutical development - European








