
Quantitative Analysis of Polysorbate 20/80 in Protein-Based Biopharmaceuticals Using A One-Pot RPLC-MS Based Platform Method

Quantification and Characterization of Polysorbate-80 in Protein Formulations

Aude Smeets - IBA

Blank Wooden Sign Board Illustration Stock Vector Image Art
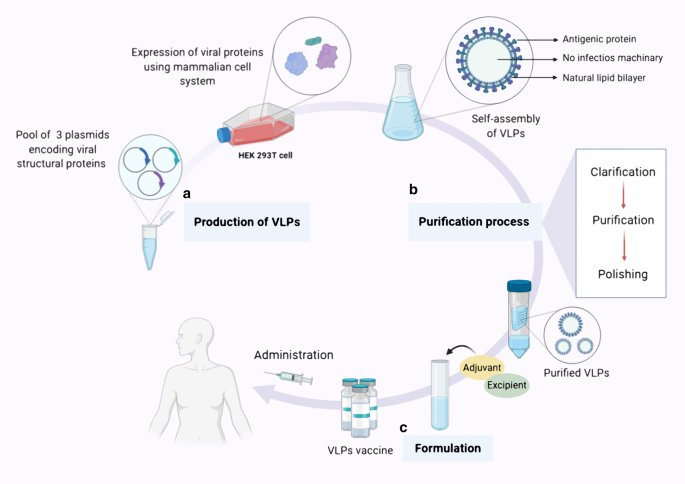
Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers, Journal of Nanobiotechnology
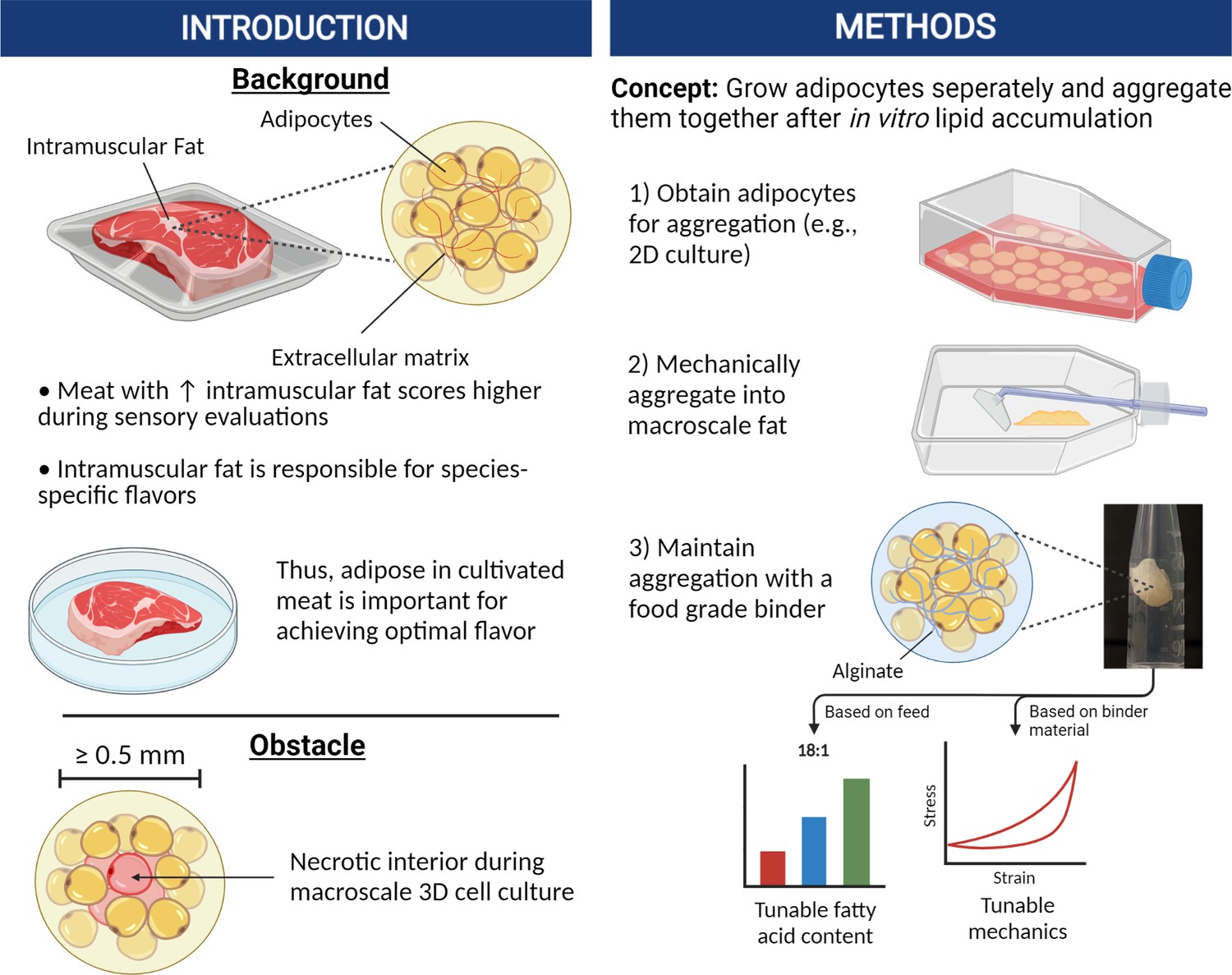
Aggregating in vitro-grown adipocytes to produce macroscale cell-cultured fat tissue with tunable lipid compositions for food applications

An Efficient UV-Based Method For The Assessment Of Oleic, 42% OFF
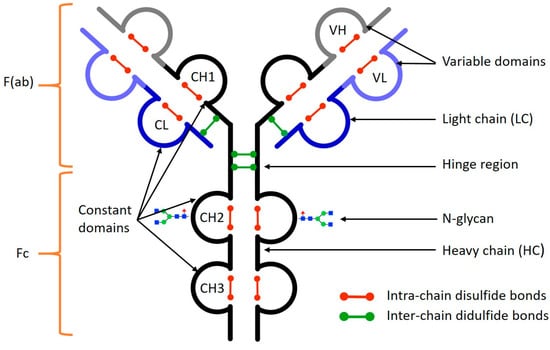
Separations, Free Full-Text

Methods Matter: Standard Production Platforms for Recombinant AAV Produce Chemically and Functionally Distinct Vectors: Molecular Therapy Methods & Clinical Development
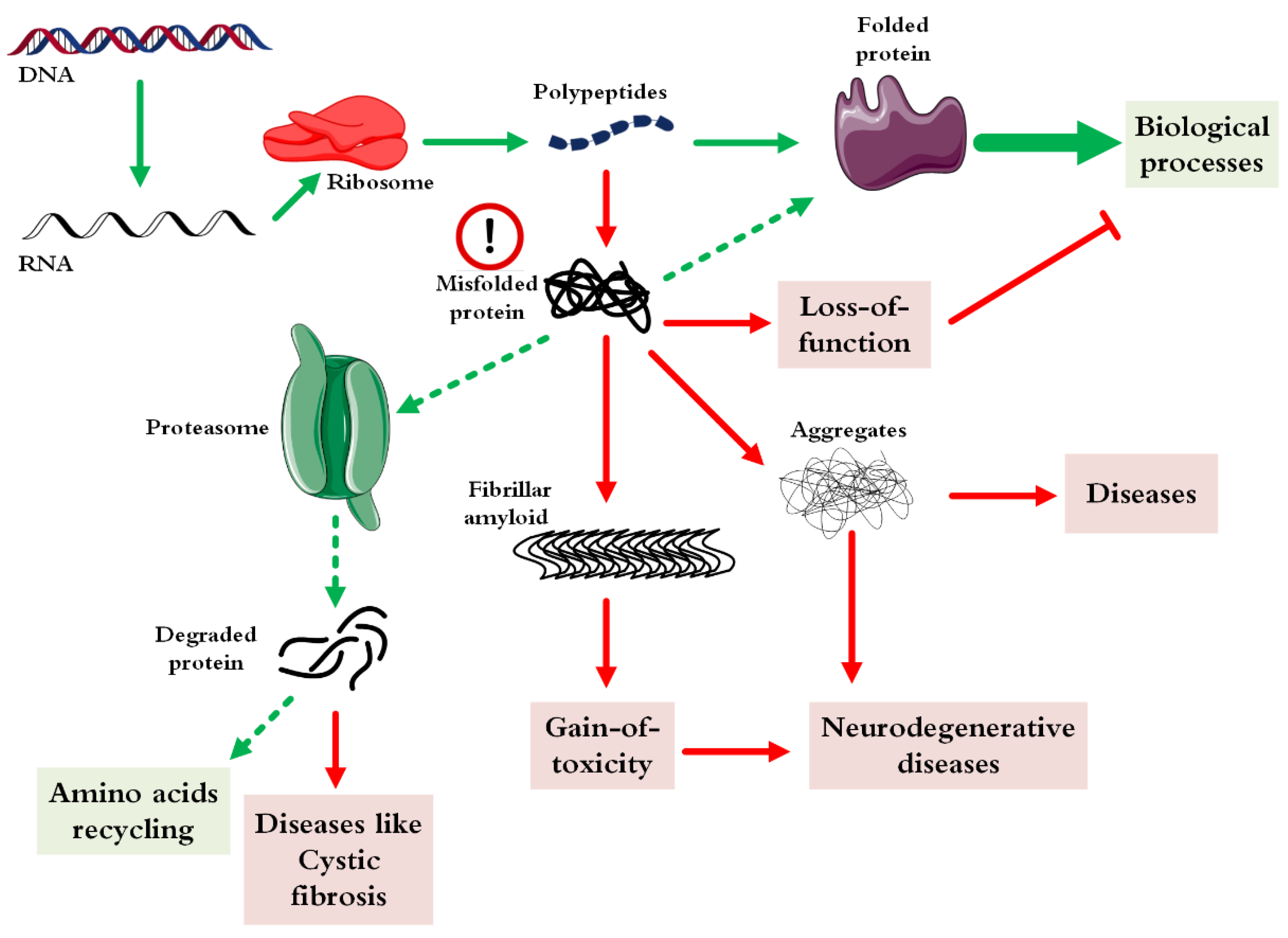
Pharmaceutics, Free Full-Text

Full article: A Platform analytical method for intact polysorbates in protein-containing biopharmaceutical products via HPLC-CAD

Fatty acid composition analysis in polysorbate 80 with high

An Efficient UV-Based Method For The Assessment Of Oleic, 42% OFF

Fast analysis of antibody-derived therapeutics by automated multidimensional liquid chromatography – Mass spectrometry - ScienceDirect

Paula Meleady (Eds.) ) Heterologous Protein Produc (B-Ok - CC), PDF, Dna Methylation

Improving Prediction of Free Fatty Acid Particle Formation in Biopharmaceutical Drug Products: Incorporating Ester Distribution during Polysorbate 20 Degradation








