Buffer, pH control, acid-base balance, buffer solutions
Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Ions are atoms or molecules that have lost or gained one or more electrons. An example of a common buffer is a solution of acetic acid (CH3COOH) and sodium
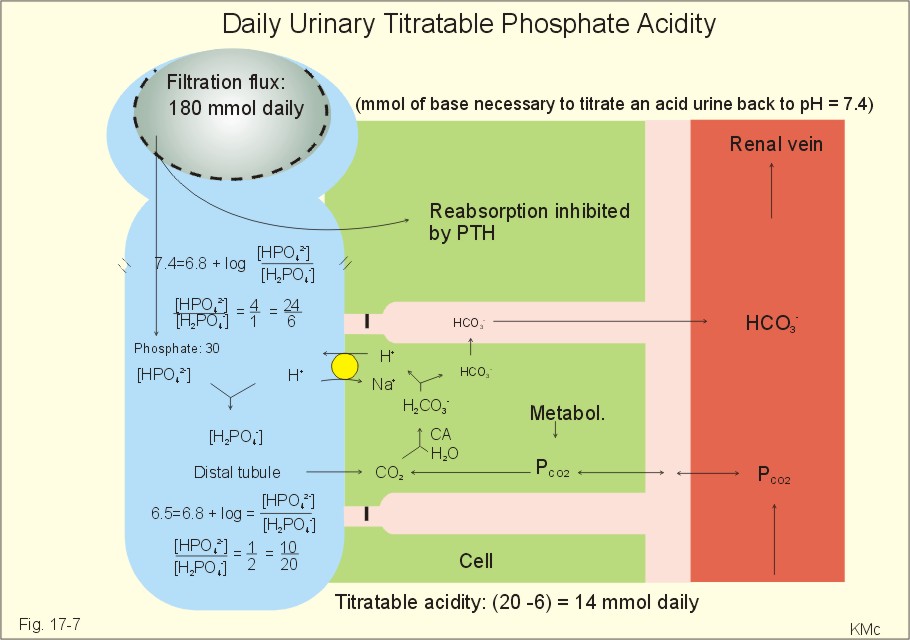
New Human Physiology Ch 17

17.2: Buffer Solutions - Chemistry LibreTexts
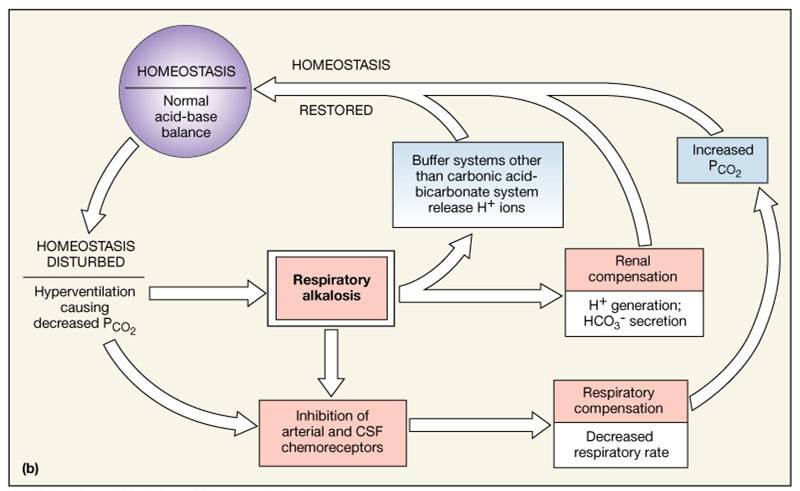
Electrolyte Fluid Balance

Properties of buffers (video), Buffers
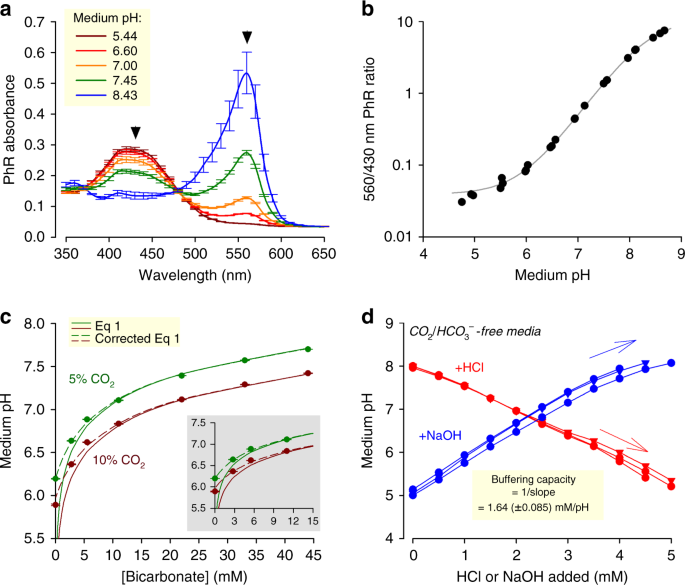
Evidence-based guidelines for controlling pH in mammalian live-cell culture systems
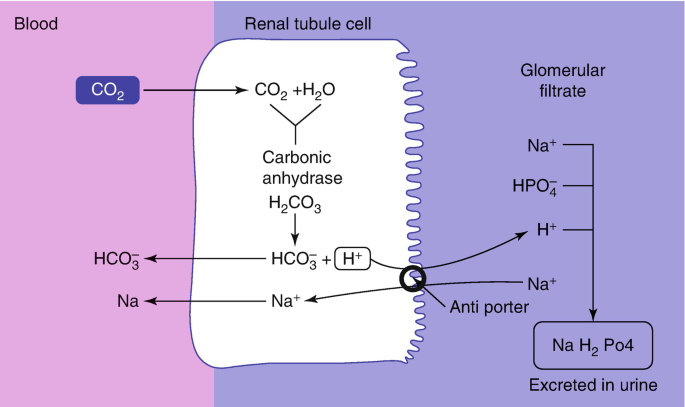
Acid-Base Balance
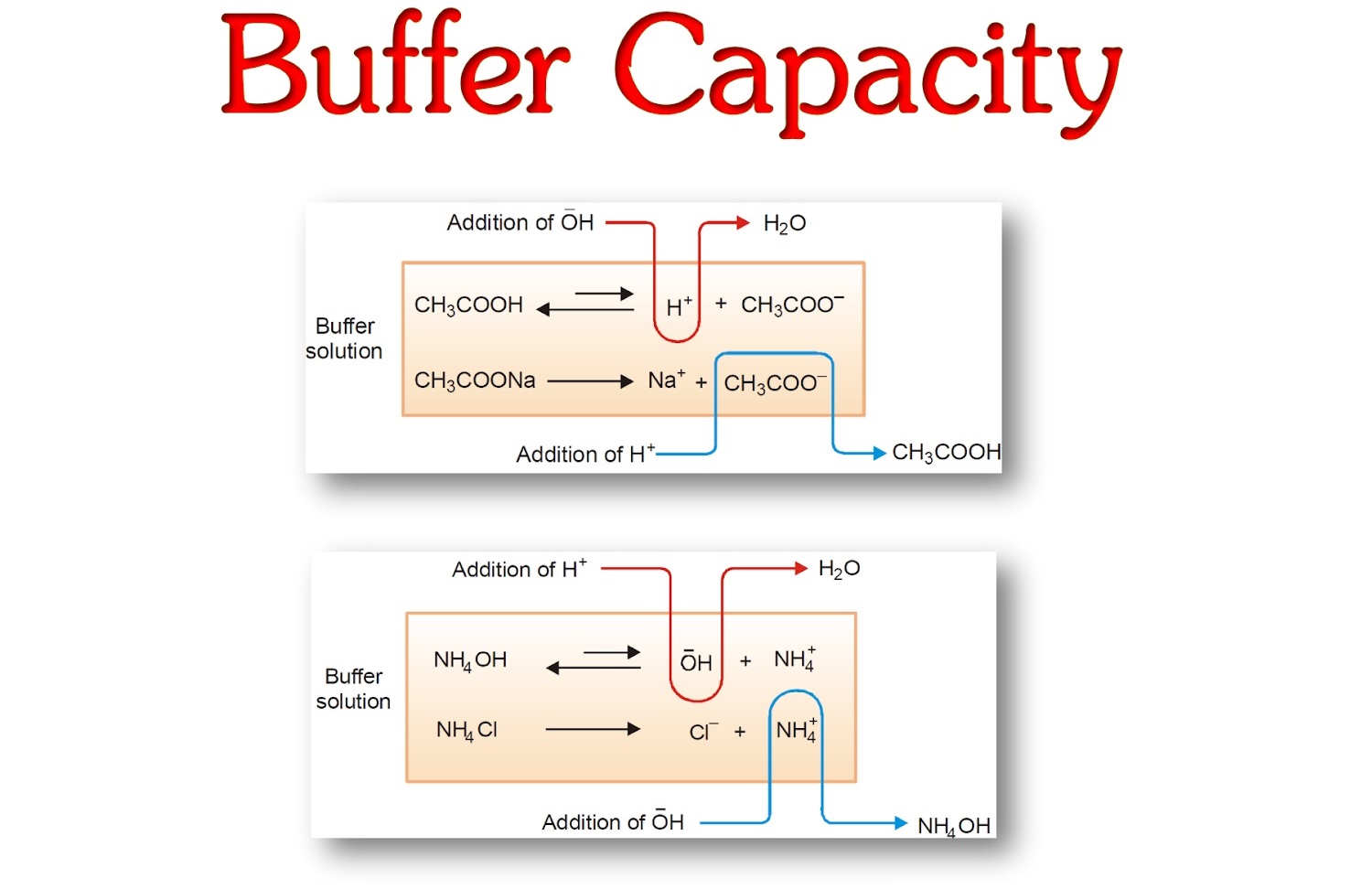
13 Enigmatic Facts About Buffer Capacity
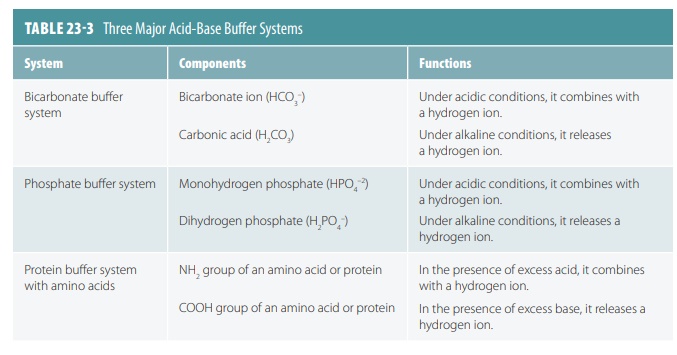
Acid-Base Balance
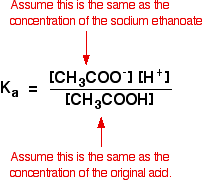
buffer solutions

Acid base regulation

Buffer Solution - Acidic and Basic Buffers, Preparations, Examples

Introduction to Buffer System, Regulation of pH, Acid Base Balance
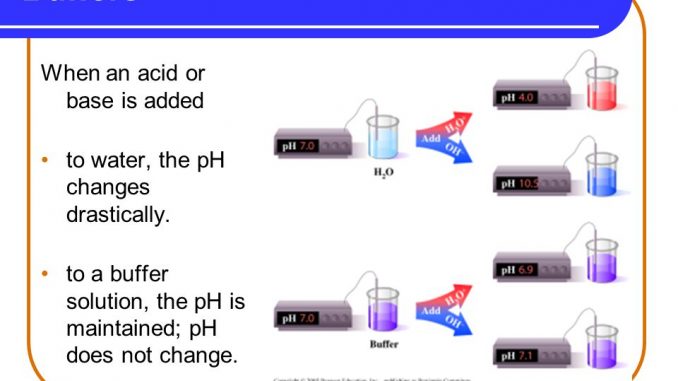
Buffer, buffering capacity, properties of good buffer and role of buffer in vitro and in vivo - Online Biology Notes

Brilliant buffers, Feature

Buffer solution - Wikipedia