
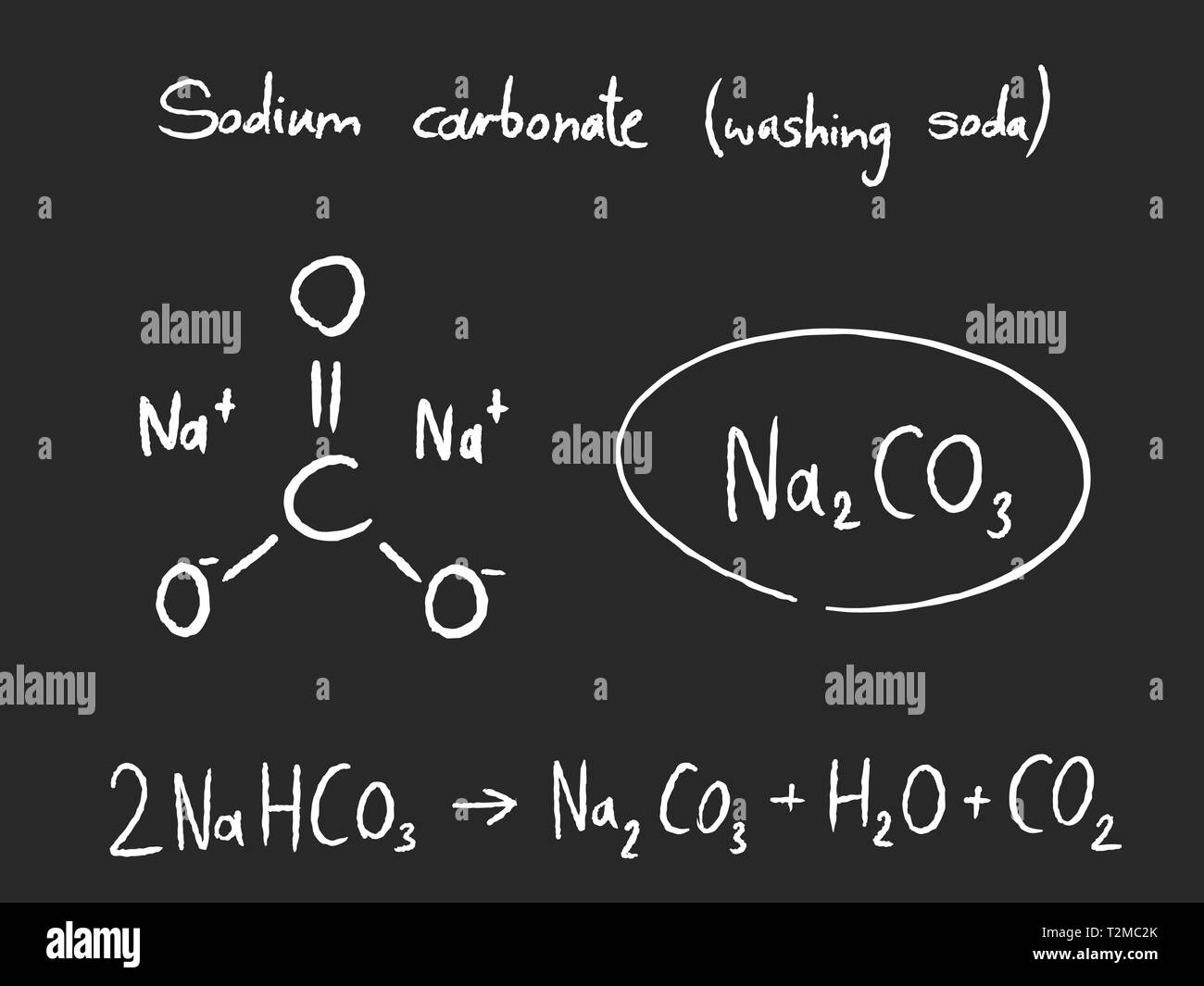
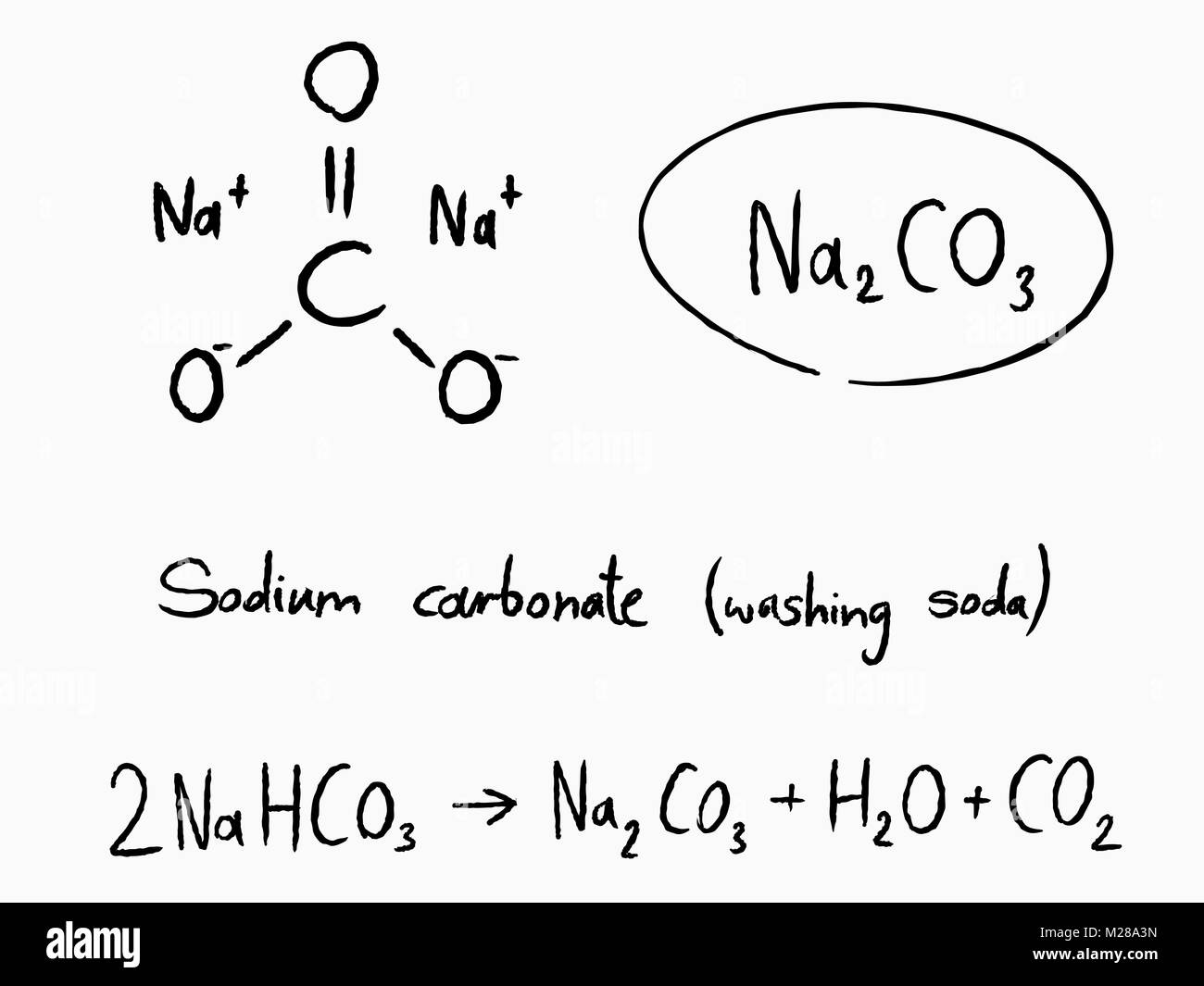
Sodium Carbonate (Washing Soda), Na2CO3⋅10H2O Sodium carbonate
Sodium Carbonate (Washing Soda), Na2CO3⋅10H2O
Sodium carbonate is generally prepared by Solvay Process. In this process, advantage is taken of the low solubility of sodium hydrogencarbonate whereby it gets precipitated in the reaction of sodium chloride with ammonium hydrogencarbonate. The latter is prepared by passing CO2 to a concentrated solution of sodium chloride saturated with ammonia, where ammonium carbonate followed by ammonium hydrogencarbonate are formed. The equations for the complete process may be written as :
2NH3+H2O+CO2→(NH4)2CO3(NH4)2CO3+H2O+CO2→2NH4HCO3NH4HCO3+NaCl→NH4Cl+NaHCO3
Sodium hydrogencarbonate crystal separates. These are heated to give sodium carbonate.
Video solution 1: Sodium Carbonate (Washing Soda), Na2CO3⋅10H2O
Sodium carbonate is generally prepared by Solvay Process. In this process, advantage is taken of the low solubility of sodium hydrogencarbonate whereby it gets precipitated in the reaction of sodium chloride with ammonium hydrogencarbonate. The latter is prepared by passing CO2 to a concentrated solution of sodium chloride saturated with ammonia, where ammonium carbonate followed by ammonium hydrogencarbonate are formed. The equations for the complete process may be written as :
2NH3+H2O+CO2→(NH4)2CO3(NH4)2CO3+H2O+CO2→2NH4HCO3NH4HCO3+NaCl→NH4Cl+NaHCO3
Sodium hydrogencarbonate crystal separates. These are heated to give sodium carbonate.

All Natural Washing Soda 1 Gallon
Sodium carbonate hi-res stock photography and images - Alamy

Sodium Carbonate, Na2CO3
Sodium carbonate - Wikipedia
What is the purpose of using sodium carbonate to neutralize hydrochloric acid? - Quora
Sodium carbonate (washing soda) - chemistry lesson. Science education Stock Vector Image & Art - Alamy

Sodium Carbonate Monohydrate from ELCO

Earthborn Elements Washing Soda (1 Gallon), Soda Ash, Sodium Carbonate, Non-Toxic Laundry Booster : Grocery & Gourmet Food

What Is Sodium Carbonate?

40 Days Crash Course for JEE Main Chemistry 9313199319, 9789313199311

40 Days Crash Course for JEE Main Chemistry 9313199319, 9789313199311
Sodium carbonate also known as soda ash and washing soda is an alkaline product is used in production of detergents, water softening, and cooking

Sodium Carbonate, Soda Ash (NaCO3)
Appearance: White Solid Sodium Carbonate uses: An intermediate for the production or for the treatment of a wide variety of products in the chemicals

Sodium Carbonate (Soda Ash Dense) [Na2CO3] [CAS_497-19-8] 99.6+%, White Solid (50 Lb Bag) by Wintersun Chemical

6132-02-1・Sodium Carbonate Decahydrate・191-01545[Detail Information], [Common Chemicals & Lab Tools]

Sodium Sesquicarbonate [Na2CO3.NaHCO3 .2H2O] [CAS_533-96-0] White Needle (50 Lbs Bag) by Wintersun Chemical: : Industrial & Scientific









