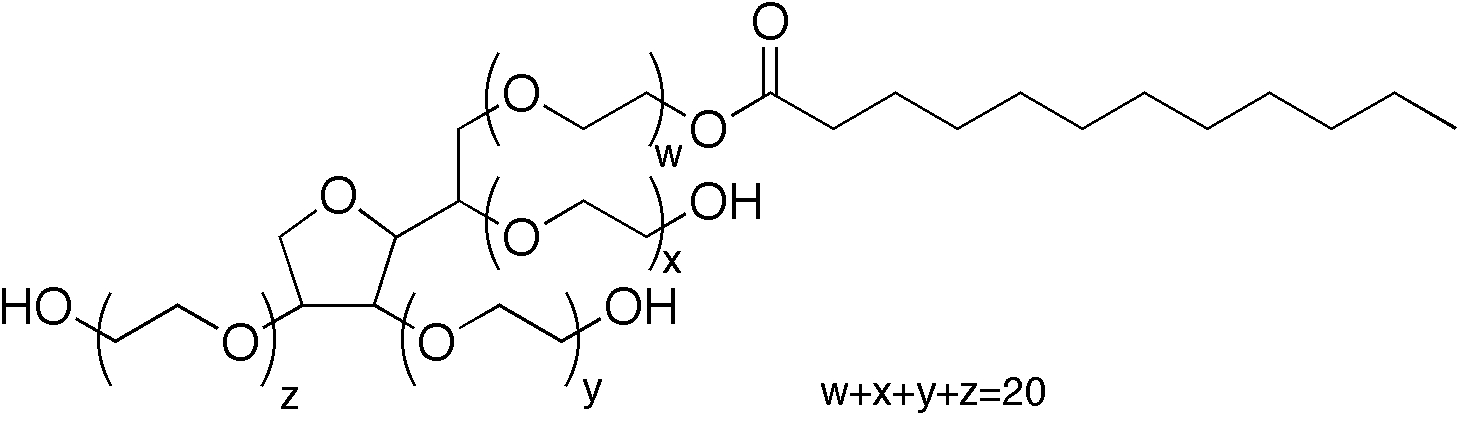
Characterization and Stability Study of Polysorbate 20 in Therapeutic Monoclonal Antibody Formulation by Multidimensional Ultrahigh-Performance Liquid Chromatography–Charged Aerosol Detection–Mass Spectrometry

Mixed-mode and reversed-phase liquid chromatography–tandem mass spectrometry methodologies to study composition and base hydrolysis of polysorbate 20 and 80 - ScienceDirect
View of ANALYSIS OF POLYSORBATE 80 SOLUTION STABILITY UNDER STRESS CONDITIONS TO ENSURE ITS QUALITY AS A BIOPHARMACEUTICAL EXCIPIENT

Table 1 from Understanding Particle Formation: Solubility of Free Fatty Acids as Polysorbate 20 Degradation Byproducts in Therapeutic Monoclonal Antibody Formulations.

Screening of Polysorbate-80 Composition by High Resolution Mass Spectrometry with Rapid H/D Exchange

Degradation Mechanisms of Polysorbate 20 Differentiated by 18O-labeling and Mass Spectrometry

A Mechanistic Understanding of Monoclonal Antibody Interfacial Protection by Hydrolytically Degraded Polysorbate 20 and 80 under IV Bag Conditions

Mixed-mode chromatography in pharmaceutical and biopharmaceutical applications – topic of research paper in Chemical sciences. Download scholarly article PDF and read for free on CyberLeninka open science hub.

Characterization and Stability Study of Polysorbate 20 in Therapeutic Monoclonal Antibody Formulation by Multidimensional Ultrahigh-Performance Liquid Chromatography–Charged Aerosol Detection–Mass Spectrometry

A Mechanistic Understanding of Monoclonal Antibody Interfacial Protection by Hydrolytically Degraded Polysorbate 20 and 80 under IV Bag Conditions

Screening of Polysorbate-80 Composition by High Resolution Mass Spectrometry with Rapid H/D Exchange

Process for reducing subvisible particles in a pharmaceutical formulation Patent Grant Bak , et al. July 9, 2 [Regeneron Pharmaceuticals, Inc.]
PDF) HPLC for Characterization and Quality Control of Therapeutic Monoclonal Antibodies