FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium Iomeron (iomeprol injection) to Address Supply Shortages
Bracco Diagnostics Inc. has announced that the U.S. Food and Drug Administration (FDA) granted import discretion of Iomeron (iomeprol injection) into the U.S. to address the ongoing iodinated contrast media shortage. The product addresses the need for the most advanced diagnostic imaging standards and will be temporarily available in the U.S. market starting at the end of August, 2022.
articles • APPLIED RADIOLOGY

Federal Register :: Authorization of Emergency Use of an In Vitro Diagnostic Device in Response to an Outbreak of Mpox; Availability

Efficacy and Safety of Gadopiclenol with Contrast-enhanced MRI of the Central Nervous System Published

Siemens Announces FDA Clearance of Three New Cios Mobile C-arms

Contrast Media Imaging Technology News

Federal Register :: Authorization of Emergency Use of an In Vitro Diagnostic Device in Response to an Outbreak of Mpox; Availability

Orthopedic Institute Selects Konica's Complete Digital X-ray Suite

Home Imaging Technology News - 阿根廷vs乌拉圭直播
Home Imaging Technology News - 阿根廷vs乌拉圭直播

EHR Interventions for Contrast Media Shortage Impact CT Utilization

InfiMed's Completes Development of i5 Platform, Launches new DR and RF/DSA Products
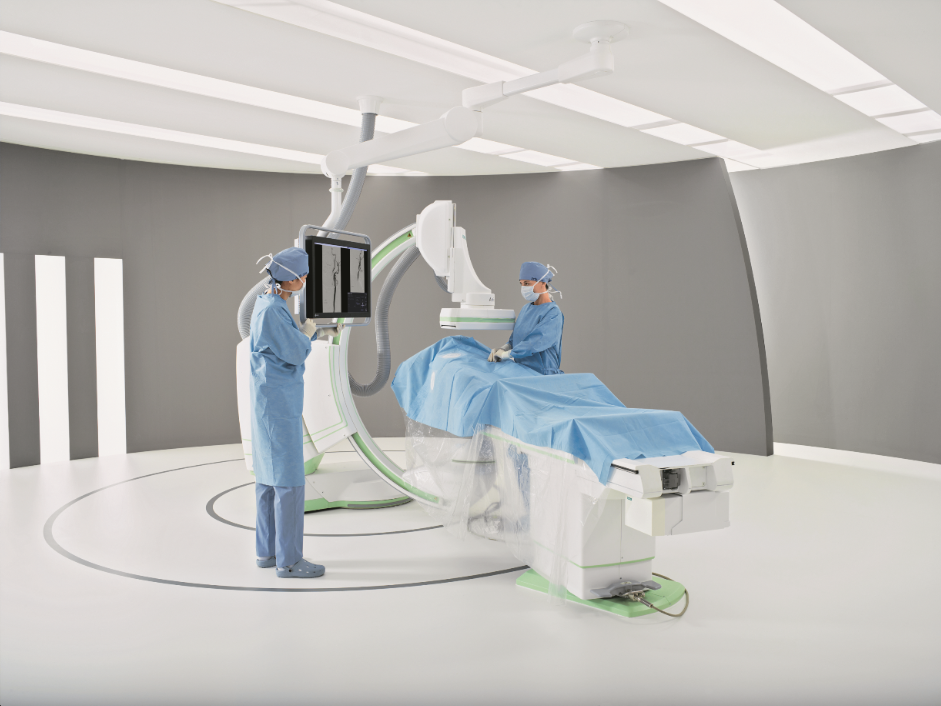
Siemens Announces FDA Clearance of Artis one Angiography System
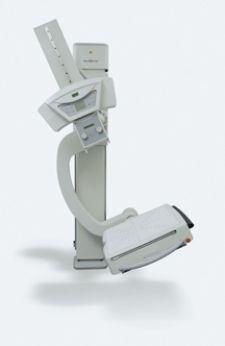
Kodak Receives FDA Clearance for New DR System