
Difference Between Carbon and Graphite Compare the Difference Between Similar Terms
The key difference between carbon and graphite is that the carbon is a chemical element whereas the graphite is an allotrope of carbon. Carbon and graphite

Graphene & Graphite - How Do They Compare? – Graphenea

Diamond and graphite are both made of carbon but very different properties. They are said to be allotropes of carbon. a. What is the molecular structure of diamond and graphite? b. Explain
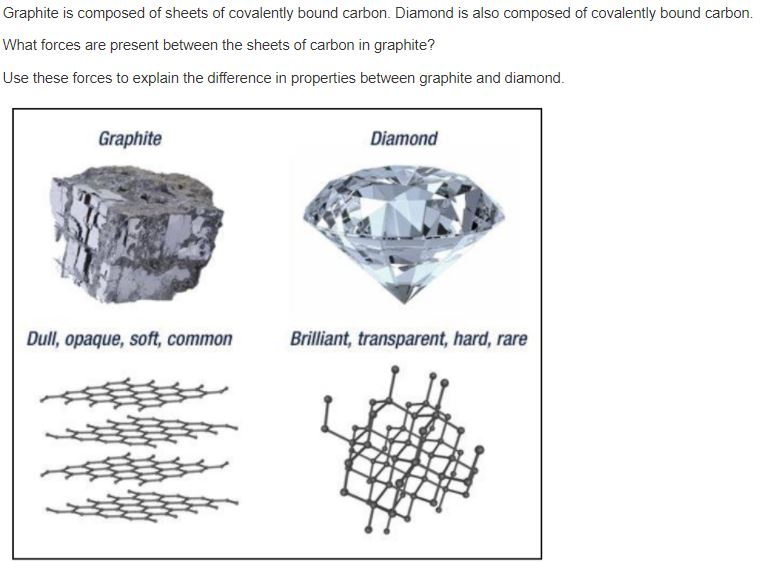
Solved Graphite is composed of sheets of covalently bound
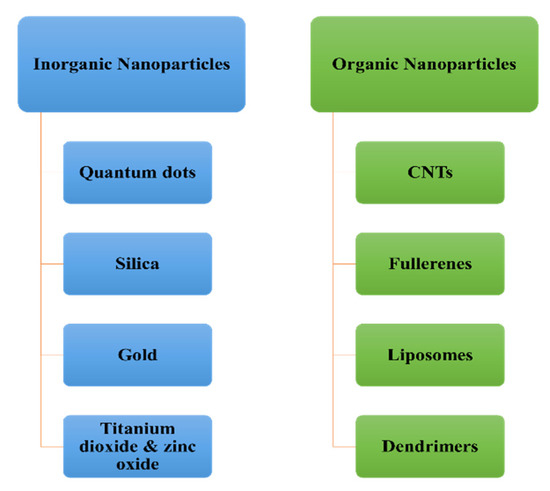
J. Compos. Sci., Free Full-Text
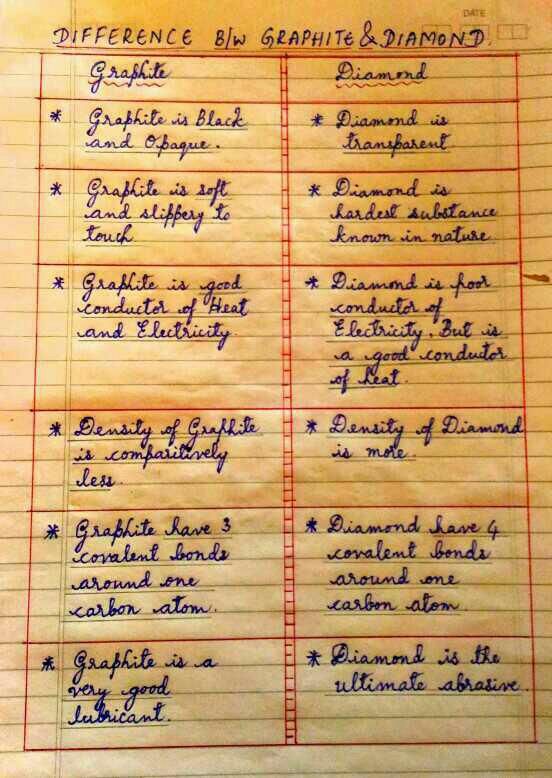
What are the difference between graphite and diamond? - EduRev Class 10 Question

The Different Allotropes Of Carbon: Graphite And Diamond – Coronet
What are the similarities between graphite and diamonds? - Quora

Carbon vs. Graphite: Know the Difference

Graphene: The World's Super Material — Linx

What is the Difference Between Allotropes and Polymorphs
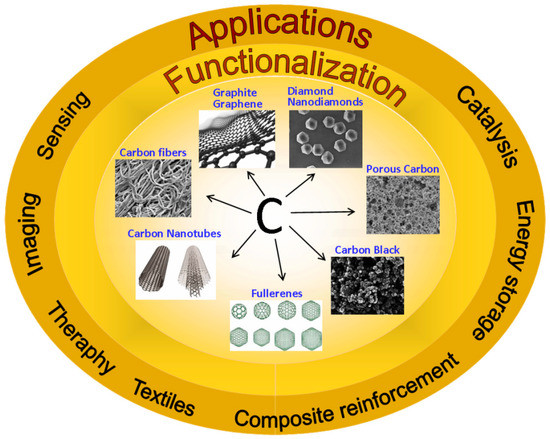
C, Free Full-Text

Graphite Flows in the U.S.: Insights into a Key Ingredient of
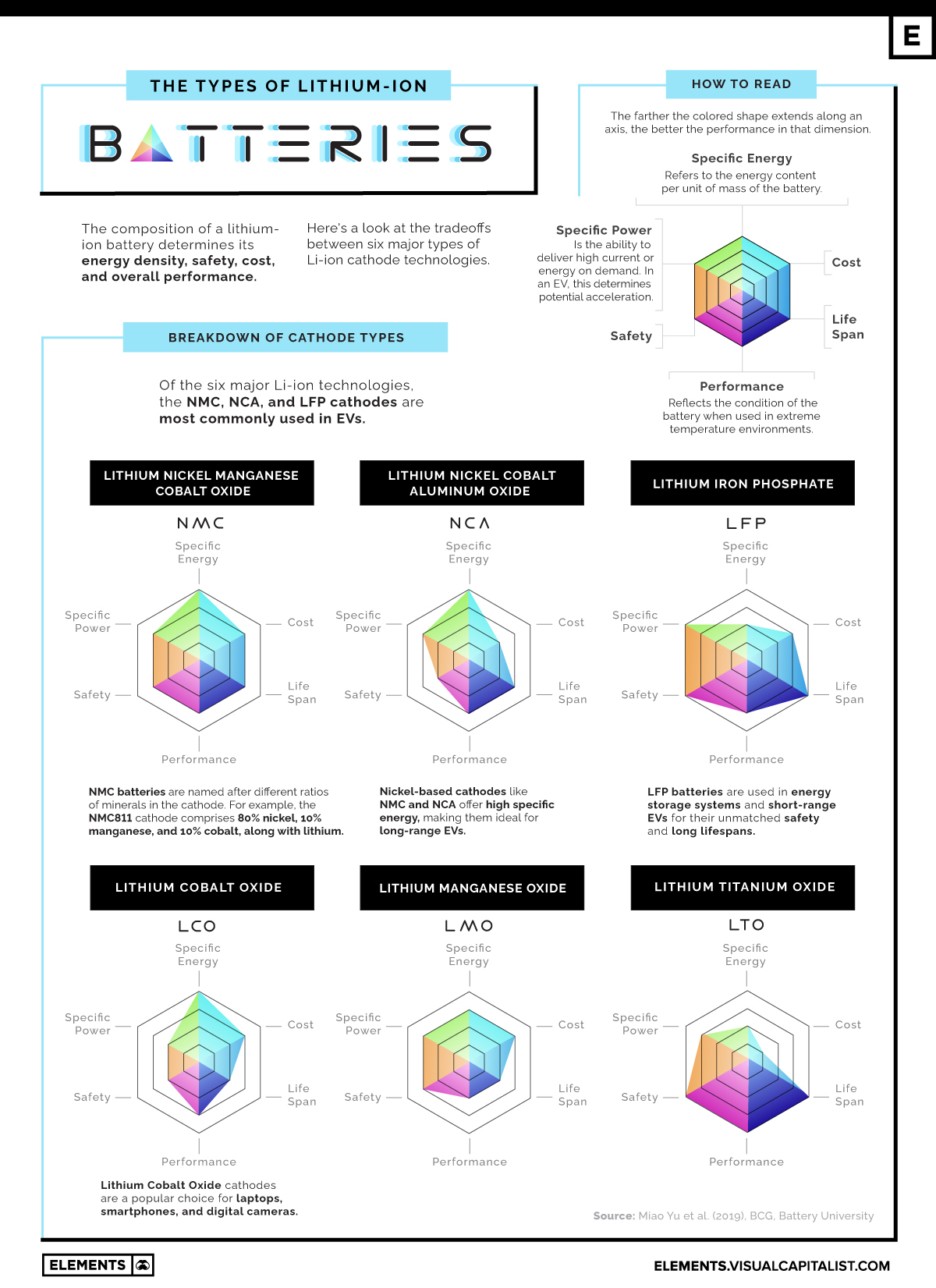
The Six Major Types of Lithium-ion Batteries: A Visual Comparison
Is carbon and graphite the same thing? - Quora
:max_bytes(150000):strip_icc()/metals-versusnonmetals-608809-v3-5b56348946e0fb0037001987.png)
The Difference Between Metals and Nonmetals









