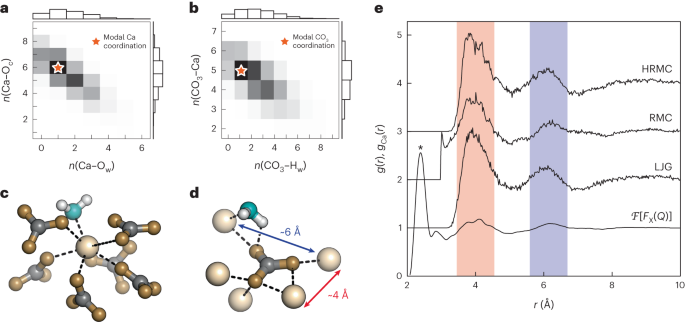
For calcium carbonate draw both the cation and the anions as standalone ions. Draw the most common Lewis structure, and do not draw alternative resonance forms.

Draw the Lewis structure for the polyatomic carbonate (co} anion: Be sure to include all resonance

Draw all significantly contributing resonance structures for the I_3^- and I_5^- polyatomic anions. Include natural bond order/hyper conjugated structures with formal charges assigned. You do not need to redraw symmetrically equivalent resonance

Soil Organic Matter Characterization by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FTICR MS): A Critical Review of Sample Preparation, Analysis, and Data Interpretation

The structure of fluorite (CaF_2) may be considered as a simple

How to draw the Lewis Dot Structure for Calcium Carbonate

Chem exam 2 Flashcards
8.24 For the carbonate ion, CO3 2−, draw all of the resonance structures. Identify which orbitals
Solved] For the carbonate ion, CO3 2−, draw all of the resonance

For magnesium hydroxide draw both the cation and the anions as standalone ions. Draw the most common Lewis structure, and do not draw alternative resonance forms.

Formal Charges and Resonance

Tips to Draw All Resonance Structures of Cations & Anions

Operando Characterization of Organic Mixed Ionic/Electronic Conducting Materials

Antacid tablets commonly contain calcium carbonate and/or magnesium hydroxide. Draw the Lewis structures for calcium carbonate and magnesium hydroxide.

Resonance Structures for CO3 2- (Carbonate ion)

Formal Charges and Resonance