FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium
Bracco Diagnostics Inc. has announced that the U.S. Food and Drug Administration (FDA) granted import discretion of Iomeron (iomeprol injection) into the U.S. to address the ongoing iodinated contrast media shortage. The product addresses the need for the most advanced diagnostic imaging standards and will be temporarily available in the U.S. market starting at the end of August, 2022.

Whale Imaging Launches New G-Arm Duo Imaging System

Bracco Announces FDA Approval of Gadopiclenol Injection, a New Macrocyclic High-Relaxivity Gadolinium-Based Contrast Agent which will be commercialized as VUEWAY™ (gadopiclenol) injection and VUEWAY™ (gadopiclenol) Pharmacy Bulk Package by Bracco

Contrast Media Imaging Technology News
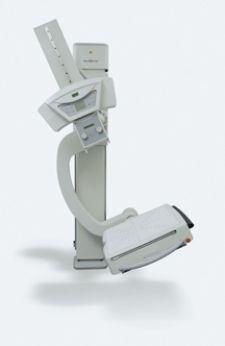
Kodak Receives FDA Clearance for New DR System

Simplifying Computed Tomography Angiography (CTA)
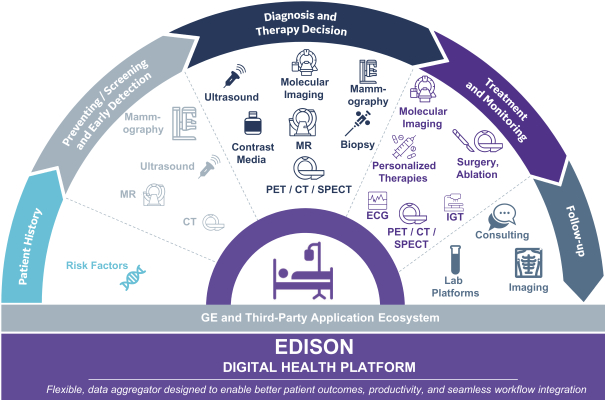
S-1

Surgical C-Arm Combines Advantages of Other Tables
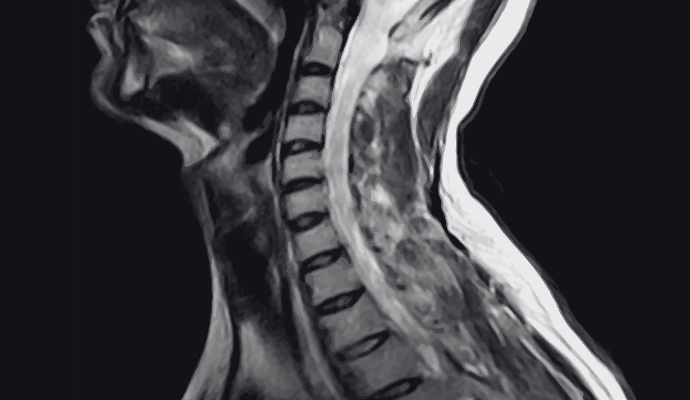
FDA Approves New MRI Contrast Agent for Nervous System Imaging
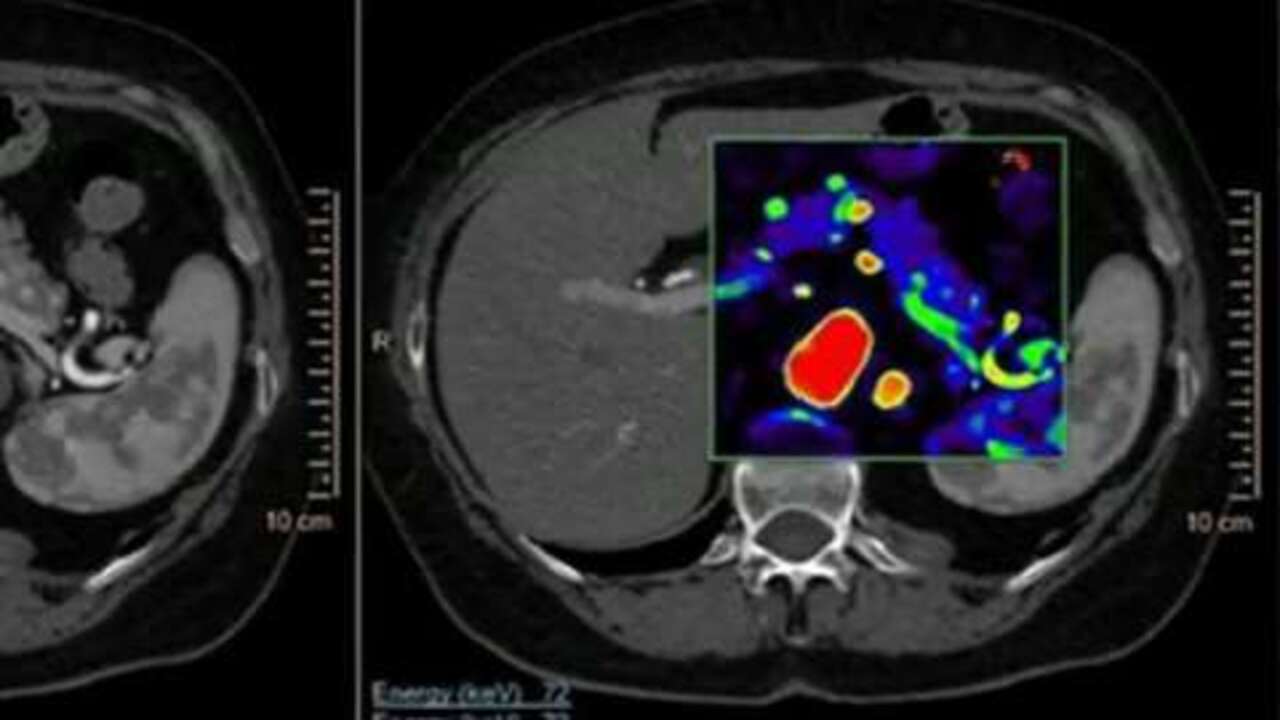
Imaging Technology News

PDF) Use of Intravenous Gadolinium-Based Contrast Media in Patients With Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation