
Sodium Acetate(CH3COONa) - Structure, Properties, Preparations, Uses, Important questions, FAQs of sodium acetate.
Sodium Acetate(CH3COONa)- Sodium acetate is the salt of acetic acid and sodium hydroxide. It is widely used across a number of industrial sectors. It is hygroscopic in nature and easily soluble in water. It is usually odourless but when heated to decomposition it smells like vinegar or acetic acid. To learn more about Sodium Acetate Preparation, Properties, Uses and FAQs, Visit BYJU’S for a detailed explanation.
Sodium Acetate(CH3COONa)- Sodium acetate is the salt of acetic acid and sodium hydroxide. It is widely used across a number of industrial sectors. It is hygroscopic in nature and easily soluble in water. It is usually odourless but when heated to decomposition it smells like vinegar or acetic acid. To learn more about Sodium Acetate Preparation, Properties, Uses and FAQs, Visit BYJU’S for a detailed explanation.

General Principles and Strategies for Salting-Out Informed by the Hofmeister Series
sodium acetate - Overview, Structure, Properties & Uses
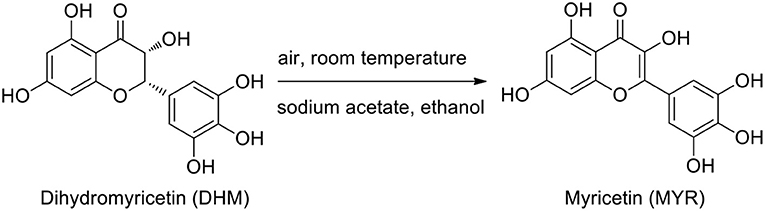
Frontiers Evaluation of General Synthesis Procedures for Bioflavonoid–Metal Complexes in Air-Saturated Alkaline Solutions

Review on sodium acetate trihydrate in flexible thermal energy storages: Properties, challenges and applications - ScienceDirect

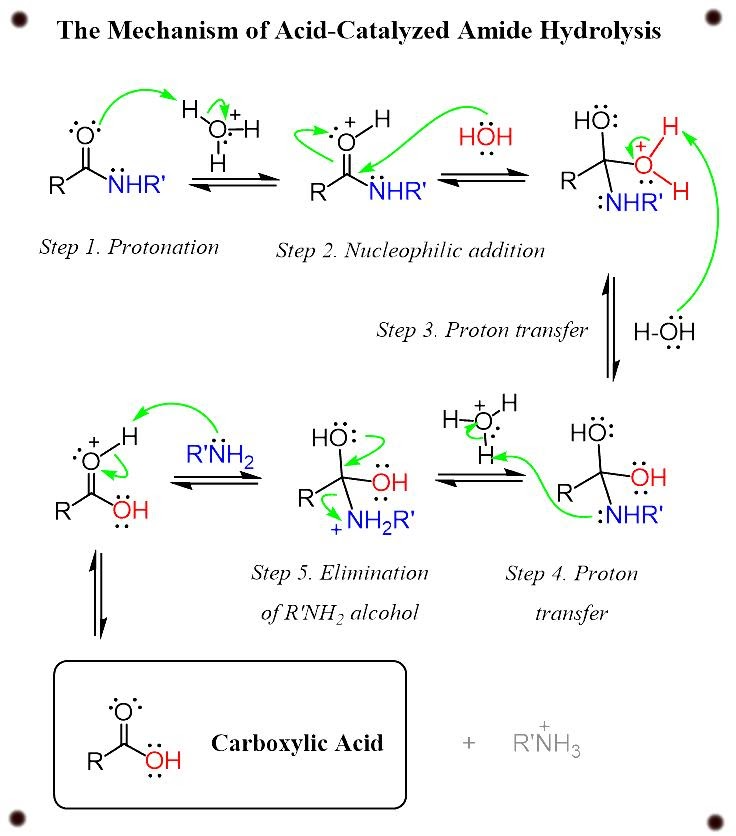
The pH of an equal molar acetic acid/sodium acetate buffer is 4.74. Draw a molecular representation of a small portion of this buffer solution. (You may omit the water molecules.) Draw another
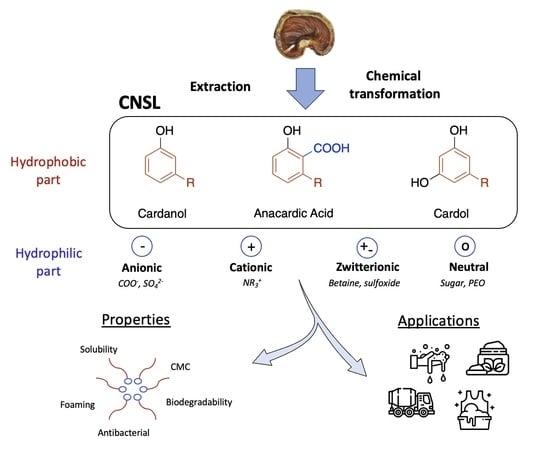
Molecules, Free Full-Text

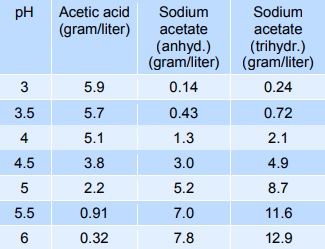
Important role of sodium acetate
Sodium acetate
Is sodium acetate classified as an acid or a base? - Quora









